|
Suppliers for CAS
7689-03-4
|
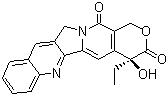
Properties | | CAS |
7689-03-4 | | Formula |
C20H16N2O4 |
|
|
25 Registered suppliers
Simagchem Corporation
P.R.China
Xiamen Hisunny Chemical Co., Ltd.
P.R.China
Molecular Formula : C20H16N2O4
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Molecular Formula : C20H16N2O4
Hangzhou Keying Chem Co., Ltd.
P.R.China
Molecular Formula : C20H16N2O4
H&Z Industry Co.,Ltd
P.R.China
Molecular Formula : C20H16N2O4
Hangzhou Meite Industry Co., Ltd (Hangzhou Meite Chemical Co., Ltd)
P.R.China
Molecular Formula : C20H16N2O4
Leap Chem Co., Ltd
P.R.China
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
Regent Science Industry Limited
P.R.China
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
New Natural Biotechnology Co.,Ltd.
P.R.China
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
Hangzhou Zhongqi Chem Co., Ltd
P.R.China
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
Nantong Xindao Biotech Ltd
P.R.China
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
Zehao Industry Co., Ltd.
P.R.China
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
Hangzhou Lingrui Chemical Co., Ltd.
P.R.China
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
Carbone Scientific Co., Ltd.
U.K.
Molecular Formula: C20H16N2O4 Molecular Weight: 348.35 Hazard Symbols: T,Xi,Xn UN Number: UN 1544 6.1/PG 3 WGKGermany: 3 PackingGroup: III HS Code: 29399990
More details are to be found here
Ambeed, Inc.
USA
Purity : 98% Smile code : [C@@]5(C2=C(C(N1CC4=C(C1=C2)N=C3C=CC=CC3=C4)=O)COC5=O)(CC)O
MDL Number : MFCD00081076
MolFormula : C20H16N2O4
MolWeight : 348.3521
Available in stock : 1384.784
More details are to be found here
BLD Pharmatech Ltd
P.R.China
Smile code: [C@@]5(C2=C(C(N1CC4=C(C1=C2)N=C3C=CC=CC3=C4)=O)COC5=O)(CC)O
MDL Number:
MFCD00081076
Purity :
98%
Available in stock:
747.782 g
More details are to be found here
Xiamen Equation Chemical Co.,Ltd
P.R.China
Smile code: [C@@]5(C2=C(C(N1CC4=C(C1=C2)N=C3C=CC=CC3=C4)=O)COC5=O)(CC)O
MDL Number:
MFCD00081076
Purity :
98%
Available in stock:
747.782 g
More details are to be found here
Smile code: [C@@]5(C2=C(C(N1CC4=C(C1=C2)N=C3C=CC=CC3=C4)=O)COC5=O)(CC)O
MDL Number:
MFCD00081076
Purity :
98%
Available in stock:
747.782 g
More details are to be found here
AK Scientific, Inc.
USA
Smile code: [C@@]5(C2=C(C(N1CC4=C(C1=C2)N=C3C=CC=CC3=C4)=O)COC5=O)(CC)O
MDL Number:
MFCD00081076
Purity :
98%
Available in stock:
747.782 g
More details are to be found here
Chemos GmbH & Co. KG
Germany
Smile code: [C@@]5(C2=C(C(N1CC4=C(C1=C2)N=C3C=CC=CC3=C4)=O)COC5=O)(CC)O
MDL Number:
MFCD00081076
Purity :
98%
Available in stock:
747.782 g
More details are to be found here
BuGuCh & Partners
Germany
Product Name: (+)-Camptothecin
CAS: 7689-03-4
MF: C20H16N2O4
MW: 348.35
EINECS: 444-280-6
Mp: 260 °C (Dec.)(Lit.)
Storage Temp.: 2-8°C
Solubility: Chloroform/Methanol (4:1): 4 Mg/Ml
Form: Solid
Color: Yellow
Water Solubility: Insoluble
Merck: 1735
Chemical Properties light yellow crystal powde
Usage :antineoplastic
Usage : Antitumor agent;Topoisomerase I inhibitor
Usage: 10-hydroxycamptothecine precursor, topoisomerase inhibitor, binds irreversibly to DNA-topoisomerase I complex
Usage :Antitumor alkaloid. Binds irreversible to the DNA-topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. A cytotoxic antitumor agent
Biological Activity: Cytotoxic plant alkaloid with antitumor properties; prototypic DNA topoisomerase I inhibitor.
Product Name: (+)-Camptothecin
CAS: 7689-03-4
MF: C20H16N2O4
MW: 348.35
EINECS: 444-280-6
Mp: 260 °C (Dec.)(Lit.)
Storage Temp.: 2-8°C
Solubility: Chloroform/Methanol (4:1): 4 Mg/Ml
Form: Solid
Color: Yellow
Water Solubility: Insoluble
Merck: 1735
Chemical Properties light yellow crystal powde
Usage :antineoplastic
Usage : Antitumor agent;Topoisomerase I inhibitor
Usage: 10-hydroxycamptothecine precursor, topoisomerase inhibitor, binds irreversibly to DNA-topoisomerase I complex
Usage :Antitumor alkaloid. Binds irreversible to the DNA-topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. A cytotoxic antitumor agent
Biological Activity: Cytotoxic plant alkaloid with antitumor properties; prototypic DNA topoisomerase I inhibitor.
Product Name: (+)-Camptothecin
CAS: 7689-03-4
MF: C20H16N2O4
MW: 348.35
EINECS: 444-280-6
Mp: 260 °C (Dec.)(Lit.)
Storage Temp.: 2-8°C
Solubility: Chloroform/Methanol (4:1): 4 Mg/Ml
Form: Solid
Color: Yellow
Water Solubility: Insoluble
Merck: 1735
Chemical Properties light yellow crystal powde
Usage :antineoplastic
Usage : Antitumor agent;Topoisomerase I inhibitor
Usage: 10-hydroxycamptothecine precursor, topoisomerase inhibitor, binds irreversibly to DNA-topoisomerase I complex
Usage :Antitumor alkaloid. Binds irreversible to the DNA-topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. A cytotoxic antitumor agent
Biological Activity: Cytotoxic plant alkaloid with antitumor properties; prototypic DNA topoisomerase I inhibitor.
Zhejiang Yixin Pharmaceutical Co., Ltd.
P.R.China
Camptothecin is an alkaloid derived from the Chinese tree Camptotheca acuminata Decne. Camptothecin and its derivatives are unique in their ability to inhibit DNA Topoisomerase I, by stabilizing a covalent reaction intermediate termed the cleavable complex which ultimately causes tumor cell death.
In clinical settings it is widely believed that camptothecin analogs exhibited remarkable anti-tumor and anti-leukaemia activity. Topoisomerase is a basilic enzyme in the process of DNA replication responsible for the winding / unwinding of the super-coiled DNA composing the chromosomes. If the chromosomes cannot be unwound, transcription of DNA messages cannot occur so that the protein cannot be synthesized, which ultimately causes cell death.
Application of camptothecin in clinical settings is limited due to serious side effects and poor water-solubility. At present, some camptothecin analogs—either semi-synthetic or synthetic drugs based on camptothecin—have been applied as cancerous therapy such as topotecan and irinotecan, while others have obtained satisfying curative effects in clinical applications.
Evonik ( formerly Wilshire Technologies, Inc. )
USA
Camptothecin is an alkaloid derived from the Chinese tree Camptotheca acuminata Decne. Camptothecin and its derivatives are unique in their ability to inhibit DNA Topoisomerase I, by stabilizing a covalent reaction intermediate termed the cleavable complex which ultimately causes tumor cell death.
In clinical settings it is widely believed that camptothecin analogs exhibited remarkable anti-tumor and anti-leukaemia activity. Topoisomerase is a basilic enzyme in the process of DNA replication responsible for the winding / unwinding of the super-coiled DNA composing the chromosomes. If the chromosomes cannot be unwound, transcription of DNA messages cannot occur so that the protein cannot be synthesized, which ultimately causes cell death.
Application of camptothecin in clinical settings is limited due to serious side effects and poor water-solubility. At present, some camptothecin analogs—either semi-synthetic or synthetic drugs based on camptothecin—have been applied as cancerous therapy such as topotecan and irinotecan, while others have obtained satisfying curative effects in clinical applications.
Pugh & Co International S.A. - N.V.
Belgium
Camptothecin is an alkaloid derived from the Chinese tree Camptotheca acuminata Decne. Camptothecin and its derivatives are unique in their ability to inhibit DNA Topoisomerase I, by stabilizing a covalent reaction intermediate termed the cleavable complex which ultimately causes tumor cell death.
In clinical settings it is widely believed that camptothecin analogs exhibited remarkable anti-tumor and anti-leukaemia activity. Topoisomerase is a basilic enzyme in the process of DNA replication responsible for the winding / unwinding of the super-coiled DNA composing the chromosomes. If the chromosomes cannot be unwound, transcription of DNA messages cannot occur so that the protein cannot be synthesized, which ultimately causes cell death.
Application of camptothecin in clinical settings is limited due to serious side effects and poor water-solubility. At present, some camptothecin analogs—either semi-synthetic or synthetic drugs based on camptothecin—have been applied as cancerous therapy such as topotecan and irinotecan, while others have obtained satisfying curative effects in clinical applications.
Boehringer Ingelheim GmbH
Germany
Camptothecin is an alkaloid derived from the Chinese tree Camptotheca acuminata Decne. Camptothecin and its derivatives are unique in their ability to inhibit DNA Topoisomerase I, by stabilizing a covalent reaction intermediate termed the cleavable complex which ultimately causes tumor cell death.
In clinical settings it is widely believed that camptothecin analogs exhibited remarkable anti-tumor and anti-leukaemia activity. Topoisomerase is a basilic enzyme in the process of DNA replication responsible for the winding / unwinding of the super-coiled DNA composing the chromosomes. If the chromosomes cannot be unwound, transcription of DNA messages cannot occur so that the protein cannot be synthesized, which ultimately causes cell death.
Application of camptothecin in clinical settings is limited due to serious side effects and poor water-solubility. At present, some camptothecin analogs—either semi-synthetic or synthetic drugs based on camptothecin—have been applied as cancerous therapy such as topotecan and irinotecan, while others have obtained satisfying curative effects in clinical applications.
Santa Cruz Biotechnology, Inc.
USA
Camptothecin is an alkaloid derived from the Chinese tree Camptotheca acuminata Decne. Camptothecin and its derivatives are unique in their ability to inhibit DNA Topoisomerase I, by stabilizing a covalent reaction intermediate termed the cleavable complex which ultimately causes tumor cell death.
In clinical settings it is widely believed that camptothecin analogs exhibited remarkable anti-tumor and anti-leukaemia activity. Topoisomerase is a basilic enzyme in the process of DNA replication responsible for the winding / unwinding of the super-coiled DNA composing the chromosomes. If the chromosomes cannot be unwound, transcription of DNA messages cannot occur so that the protein cannot be synthesized, which ultimately causes cell death.
Application of camptothecin in clinical settings is limited due to serious side effects and poor water-solubility. At present, some camptothecin analogs—either semi-synthetic or synthetic drugs based on camptothecin—have been applied as cancerous therapy such as topotecan and irinotecan, while others have obtained satisfying curative effects in clinical applications.
Detailed information on the suppliers of
|